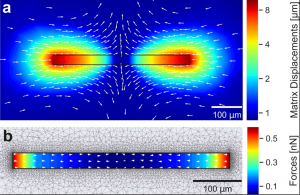
We describe a technique for simultaneous quantification of the contractile forces and cytosolic calcium dynamics of muscle fibers embedded in three-dimensional biopolymer gels. We derive a scaling law for linear elastic matrices such as basement membrane extract hydrogels (Matrigel) that allows us to measure contractile force from the shape of the relaxed and contracted muscle cell and the Young’s modulus of the matrix, without further knowledge of the matrix deformations surrounding the cell and without performing computationally intensive inverse force reconstruction algorithms. We apply our method to isolated mouse flexor digitorum brevis (FDB) fibers that are embedded in 10 mg/ml Matrigel. Upon electrical stimulation, individual FDB fibers show twitch forces of 0.37 μN ± 0.15 μN and tetanic forces (100 Hz stimulation frequency) of 2.38 μN ± 0.71 μN, corresponding to a tension of 0.44 kPa ± 0.25 kPa and 2.53 kPa ± 1.17 kPa, respectively. Contractile forces of FDB fibers increase in response to caffeine and the troponin-calcium-stabilizer Tirasemtiv, similar to responses measured in whole muscle. From simultaneous high-speed measurements of cell length changes and cytosolic calcium concentration using confocal line scanning at a frequency of 2048 Hz, we show that twitch and tetanic force responses to electric pulses follow the low-pass filtered calcium signal. In summary, we present a technically simple high speed and high throughput method for measuring contractile forces and cytosolic calcium dynamics of single muscle fibers. We expect that our method will help to reduce preparation time, costs, and the number of sacrificed animals needed for experiments such as drug testing.
Read the full article in Biophysical Journal.