The Erlangen biophysics group studies the fundamental mechanical properties of cells, tissues, and complex soft matter. We strive to understand how cells respond to their mechanical environment, how they interact with their extracellular matrix and with neighboring cells, and what mechanisms they employ for transmigration, invasion, adhesion, contraction, and cell division. We also study the collective behavior of cells in engineered microtissue such as muscle or tumor organoids. Finally, we are interested in collective behavior in animals, in particular penguins. To address these questions, our laboratory collaborates with other research groups worldwide to develop new technologies that draw from various fields, including soft matter physics, molecular cell biology, biochemistry, engineering, and applied mathematics.
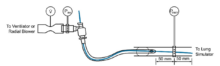
Bronchoscopy in ventilated patients severely narrows the endotracheal tube lumen and increases resistance, which can lead to hypoventilation and intrinsic PEEP build-up. These ventilation impairments depend on the geometry of the tube-bronchoscope combination, ventilator settings, and patient mechan...
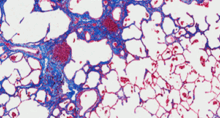
Durotaxis—cell migration along stiffness gradients—is implicated in development, repair, and disease, though its in vivo role remains unclear. We show that durotaxis actively drives disease progression in mouse models of lung fibrosis (IPF) and metastatic pancreatic cancer. In fibrosis, it guides fi...
Biofouling is a global issue that affects almost all water-based processes across various industries and significantly impacts our daily lives. Conventional biofouling treatments, such as toxic paints or antibiotics, have substantial limitations and challenges. These include the emergence of resista...
Aerophilic surfaces immersed underwater trap films of air known asplastron. Plastron has typically been considered impractical forunderwater engineering applications due to their metastable performance.Here, we describe aerophilic titanium alloy (Ti) surfaces with extendedplastron lifetimes that are...
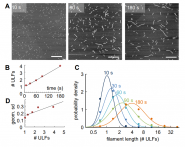
Vimentin is a highly charged intermediate filament protein that inherently forms extended dimeric coiled-coils, which serve as the basic building blocks of intermediate filaments. Under low ionic strength conditions, vimentin filaments dissociate into uniform tetrameric complexes of two anti-paralle...