3-D tissue models for investigating tumor metastasis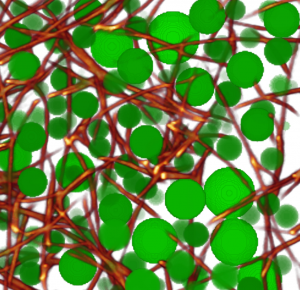
Under physiologic conditions in the organi/2015/10/19/three-dimensional-force-microscopy-of-cells-in-biopolymer-networks/sm, most cells live in a 3-D environment and not on a flat, smooth and hard 2-D plastic or glass surface. Over the past years, we have extensively used collagen-based 3-D biopolymer matrices for cell culture, and have devloped methods to characterize their structural and mechanical properties.
Licup AJ. Stress controls the mechanics of collagen networks. PNAS 2015 (PDF) Munster S. Strain history dependence of the nonlinear stress response of fibrin and collagen networks. PNAS 2013 (PDF) Lang NR. Estimating the 3D pore size distribution of biopolymer networks from directionally biased data. Biophys J 2013 (PDF) Krauss P. Parameter-Free Binarization and Skeletonization of Fiber Networks from Confocal Image Stacks. PLoS one 2012 (PDF) Mickel W. Robust Pore Size Analysis of Filamentous Networks From 3D Confocal Microscopy. Biophys J 2008 (PDF)
Traction forces in 3-D
Traction forces that cells exert on their surrounding matrix are important, for instance, for the migration of cells (such as cancer cells, or immune cells) through the connective tissue. To measure forces, we extend ideas from 2-D traction microscopy to the third dimension. In analogy to 2-D traction microscopy, 3-D tractions can be calculated by measuring the 3-D deformation field of the connective tissue matrix surrounding a cell. Of course, in 3-D things are a bit more complicated. For measuring the deformation field, one needs to record hundreds of image stacks, the matrix needs to be decorated with fiducial markers (or else needs to be directly imaged using confocal reflection microscopy), and the particle image velocimetry method of your choice needs to be able to deal with these image stacks. For computing the traction forces, the non-linear mechanical properties of the matrix need to be known, and that by itself is not entirely simple – see above.
Butler JP. Traction fields, moments, and strain energy that cells exert on their surroundings. AJP 2002(PDF) Koch TM. 3D Traction Forces in Cancer Cell Invasion. PloS one 2012 (PDF) Steinwachs J. Three-dimensional force microscopy of cells in biopolymer networks. Nature Methods 2016 (PDF)
Group dynamics in penguin colonies
 We study the collective dynamics of penguin movements with image processing technologies adapted from cell migration and particle tracking. During the antarctic winter, emperor penguins have to sustain temperatures down to -50° Celsius combined with strong winds. To conserve energy, they move close together and share their body heat (huddling). Movements inside the huddle are highly coordinated so that every penguin gets to pass the warmest zone in the center of the huddle. We study how these huddles move, and how the penguins move inside the huddle, by taking time lapse images (every 1 sec) from an elevated position, and tracking the head of every single penguin for several hours.
We study the collective dynamics of penguin movements with image processing technologies adapted from cell migration and particle tracking. During the antarctic winter, emperor penguins have to sustain temperatures down to -50° Celsius combined with strong winds. To conserve energy, they move close together and share their body heat (huddling). Movements inside the huddle are highly coordinated so that every penguin gets to pass the warmest zone in the center of the huddle. We study how these huddles move, and how the penguins move inside the huddle, by taking time lapse images (every 1 sec) from an elevated position, and tracking the head of every single penguin for several hours.
Zitterbart DP. Coordinated movements prevent jamming in an Emperor penguin huddle. PLoS one 2011 (PDF) Gerum RC. The origin of traveling waves in an emperor penguin huddle. New J Phys 2013 (PDF) Zitterbart DP. Are environmental factors responsible for changed breeding behaviour in emperor penguins? AntSci 2014 (PDF) Gerum RC. Structural organisation and dynamics in king penguin colonies. JApplPhysD (PDF) Richter S. A remote‐controlled observatory for behavioural and ecological research. Methods Ecology Evolution 2018 (PDF) Richter S. Phase transitions in huddling emperor penguins. JApplPhysD 2018 (PDF)

